1. Insects lack red blood
cells. Instead, an oxygen
carrier/protein very similar to hemoglobin is secreted directly into insect
blood. Briefly hypothesize how the
synthesis of this protein differs from that of its vertebrate relative and
describe one direct test of your hypothesis and its possible results.
A. What does the question ask?
Insects lack red
blood cells. Instead, an oxygen
carrier/protein very similar to hemoglobin is secreted directly into insect
blood . . .
The first two sentences of the question asks you to think about the synthesis of two similar proteins, one free in the blood (insects) and one contained within a red blood cell (vertebrate). While implicitly stated in the question, basic knowledge of red blood cell structure and function (from lab or lecture) is necessary to know that vertebrate hemoglobin is contained in erythrocytes:
Briefly hypothesize how the synthesis of this protein differs from that of its vertebrate relatives . . .
The question then asks you to trace the path of the insect oxygen carrier/protein during its synthesis and compare the path to hemoglobin synthesis (not secreted).
. . .and describe
one direct test of your hypothesis and its possible results.
Finally, you are asked to design an experiment to test your hypothetical pathway. Furthermore, the question asks to discuss possible results. Thus, answering this question completely requires that:
1. You recall how secreted and non-secreted proteins are synthesized,
2. You formulate a hypothesis with regards to the insect oxygen carrier, a secreted
protein,
3. You briefly describe a test, and
4. You describe possible results of the test.
All four parts are crucial to an excellent answer.
Proposing an experiment is not enough; you must discuss possible results! As long as the proposed experiment is logically based on a rational answer to the previous parts of the question, credit will be awarded even though your "facts" may be partially or completely wrong. Several experiments based on readings, lecture and lab notes, as well as your own logical creativity are possible.
2. What question is NOT
being asked?
No functional information about hemoglobin or oxygen carrier/protein is required to answer the question. Also it might be tempting to include information about the osmotic behavior of red blood cells because one lab was dedicated to the subject, but such information is not relevant to the question and should be omitted. You may be penalized for including extraneous material but more importantly doing so wastes your time.
3. What's ambiguous about
the question?
If anything doesn't make sense to you, ask the professor specific pointed questions. Don't say, "I don't understand the question." instead ask "What do you mean by __________?" or "Could you clarify __________?"
4. Now answer the question
before proceeding further.
QUESTION:
Insects lack red blood
cells. Instead, an oxygen
carrier/protein very similar to hemoglobin is secreted directly into insect
blood. Briefly hypothesize how the
synthesis of this protein differs from its vertebrate relative and describe one
direct test of your hypothesis and its possible results.
Sample
Responses: Answer
#1
|
Overview: He begins
the question by reminding himself that hemoglobin is within red blood cells
while insect protein is secreted.
Based on this fact, he traces the path of synthesis for a secreted
peptide and compares it with hemoglobin's synthesis. He concludes that the oxygen carrier is
secreted because it has a signal sequence (and possible targeting sequences)
which hemoglobin lacks. He proposed
to test for the presence of a signal sequence on the oxygen carrier by analyzing
mRNA. The oxygen carrier is expected
to have a hydrophobid end. |
ANSWER COMMENTARY
|
Because
the insect protein is secreted directly into the blood, it will be
co-translationally imported in the ER and targeted for the secretory
vesicles. Hemoglobin, on the other
hand, is a product of red bloods cells, and remains within these cells. Therefore, the insect protein will, when
it is first translated, begin with a hydrophobic signal sequence to allow it
to enter the ER. (It may also have a
second signal added to it in the ER or Golgi to target it to the secretory
vesicles.) We might be able to test
the idea that the insect protein will begin with a signal sequence while the
hemoglobin will not by isolating the genes for each in vitro and analyzing
their products. We expect the oxygen
carriers produced by insects to have a hydrophobic end and hemoglobin not to. |
The
synthesis pathways are clearly and correctly traced in the first
paragraph. The alternating structure
of the 1st 2 sentences is effective especially for answering
compare/contrast questions. The
hypothesis is basically that the insect carrier protein is secreted because
it contains a signal sequence.
Hemoglobin does not contain such a sequence, so he designed an experiment
to detect the sequence. A
good test, but unfortunately he doesn't specifically state how he intends to
do so experimentally. He could have
referred to specific experimental techniques learned in lecture. In a "brief" answer it is not
necessary to provide technical details; nevertheless, more information is
required than provided here. The
results he would expect to generate from the experiment are not well described. The results need to be more explicitly
related to the hypothesis: 1) hydrophobic end=signal sequence, 2) signal
sequence=secretion and 3) secretion=protein directly in the blood. |
A Second Example:
|
Overview: First,
the student concludes the syntheses of hemoglobin and the insect oxygen
carrier must be different because the fates of the two proteins are
different. Then, she proceeds to
detail how the oxygen carrier is synthesized, processed, and secreted using text
and diagrams. As a logical extension,
she decides to experimentally monitor the synthetic pathway of the two
proteins by a pulse-chase experiment.
The results are presented in a graph and conclusions are drawn from
the graphs to explain the differences between the two proteins. |
ANSWER COMMENTARY
|
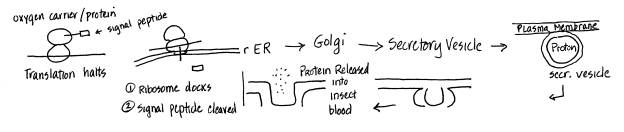
Hemoglobin
in RBC is synthesized to stay inside the RBC while the oxygen carrier/protein
in insects is synthesized to be exported.
As a result, processes of synthesis is different. The hemoglobin is synthesized in the
cytoplasm and stays in cytoplasm therefore it lacks a signal peptide. The oxygen carrier/protein will be
exported so it will have a signal protein that will associate with a stop
particle that will halt synthesis until it comes in contact with rER docking
site and translation will resume. The
signal peptide will be cleaved with signal peptidase. Then the protein will be brought to the
cis-side of the Golgi via transitory vesicles where it will be further
processed. The protein probably binds
to a receptor side on the trans side of the Golgi and a secretory vesicle is
formed with the protein inside. The
secretory vesicle moves towards the plasma membrane where it fuses with the
membrane, the protein is released into the insect blood and the secretory
vesicle membrane becomes part of the plasma membrane. Diagrams: |
The answer is laid out effectively by
starting with general knowledge. In
doing so, the reader is told what to expect from the rest of the answer; a more
detailed comparison of hemoglobin and oxygen carrier pathways. She traces the pathway of the oxygen
carrier protein synthesis from the rER-->Golgi-->secretory-->plasma
membrane-->insect blood. Some of
the details are fuzzy – e.g., “stop particle” instead of SRP, and it’s not
clear what “further processed” means.
Otherwise, a Very Good answer! Note how helpful, but how simple, a
diagram, is for organizing and expressing thoughts. |
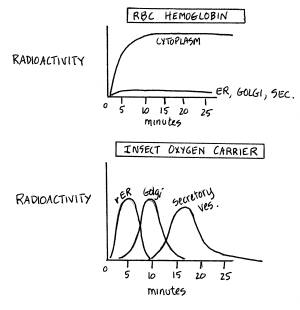
Continuing
Example 2:
|
To
observe the movement of the hemoglobin and oxygen carrier/ protein, one could
perform a "pulse-chase experiment." Radioactively labeled nucleotides would be added for five
minutes and then cells would be incubated with non-labeled nucleotides for 30
minutes and measure where the radioactivity is at certain times. The result probably would show:
Therefore,
hemoglobin stays inside the cytoplasm of the cell, and has no signal
sequence. The insect oxygen carrier/protein
goes through the rER, Golgi, secretory vesicles and is exported and has a
signal peptide that allows it into the rER. |
An excellent test for this hypothesis,
but one requiring radioactive amino acids and not nucleotides! Graphs are effective ways of
communicating data, and in this example they are especially effective for
comparing the different pathways. The exact shapes of the curves are imprecise, for idiosyncratic reasons
beyond the scope of BI250 (involving protein turnover, for example). Furthermore, the data are interpreted
correctly and related to the presence of a signal sequence and the fates of
newly synthesized proteins, either inside or outside the cell. Note also that what is being tested -- the
pathway -- is quite different than what was tested in the first Example. Given the contexts, both are reasonable
tests and equally correct answers! An Excellent answer! |
Now that you've answered the question and examined two responses in detail, review the question and critique the following 2 responses yourself in the space provided on the left.
ANSWER #3 COMMENTARY
|
Because the insects' oxygen
carrier/protein is a secretory protein, its synthesis will initially differ
in that it will produce a hydrophobic signal sequence. This sequence will then be bound by SRP
(signal recognition particle) until the ribosome migrates to the rER. Docking protein in the rER membrane will
then recognize SRT which in turn activates TRAM, forming a channel in the rER
membrane through which the nascent polypeptide may then pass. The hemoglobin of vertebrates, by
contrast, will likely be synthesized in the cytoplasm and contain no such
signal sequence for import into the rER because it is a cytoplasmic protein. A test of this hypothesis might be to
isolate the gene for the insect oxygen carrier and delete the portion which
codes for the signal sequence. This would
presumably inhibit the entry of the polypeptide into the rER and thus it
would not be secreted. |
|
Your critique might usefully lead you to edit the example, making it more accurate in the process. Use the space below for your edition –
|
ANSWER #4 |
COMMENTARY |
|
|
An obvious
difference is that the insect protein is secreted while hemoglobin is
retained within the red blood cell.
Thus, the protein (insect) at one point probably had a signal sequence
on the amino end that enabled the protein to enter the lumin of the rER. Hemoglobin lacked this type of signal
sequence and thus moved into the cytoplasm rather than into the rER. Once this insect protein went into the rER, it possibly went through
some modifications such as losing its signal sequence and/or
glycosylation. Hemoglobin had no
signal sequence to lose and would not go through glycosylation. Modification such as sulfur bridges may
occur with both. In fact, it does
with hemoglobin as it gains its quaternary structure. The insect may lack quaternary structure,
we cannot tell. However, any tertiary
or quaternary structure will take place here. The hemoglobin does not leave the cytoplasm but the insect protein
moves on through vesicles to the Golgi.
Here glycosylation will finish up as will other modifications. From here it moves to secretory vesicles
and is secreted. The hemoglobin
remains within the RBC. A way to test if the difference of location of modification does
exist between the proteins, one could get some antibodies to the protein and
inject them into appropriate cells.
These antibodies would cling on to the protein thus locating them. Most probably, the insect protein would be found in the rER, the
Golgi, or vesicles going toward the Golgi.
Secretory vesicles is another possibility. Hemoglobin will be found in the cytoplasm. If these results were found, the
difference exists. If one wanted to get more specific (i.e. test for glycosylation) one
could take both proteins and stain them with dyes that stain positive for
glycoproteins. It is possible that
the insect protein may contain oligosaccharides. It is very doubtful that hemoglobin would. |
|||