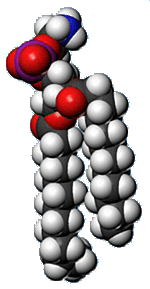
Basic membrane component is the Phospholipid molecule.
- Each molecule has a head and 2 tails.
- Heads are hydrophilic (water loving).
- Tails are hydrophobic (avoid water).
- The three most common phospholipids are distinguished by their
head groups.
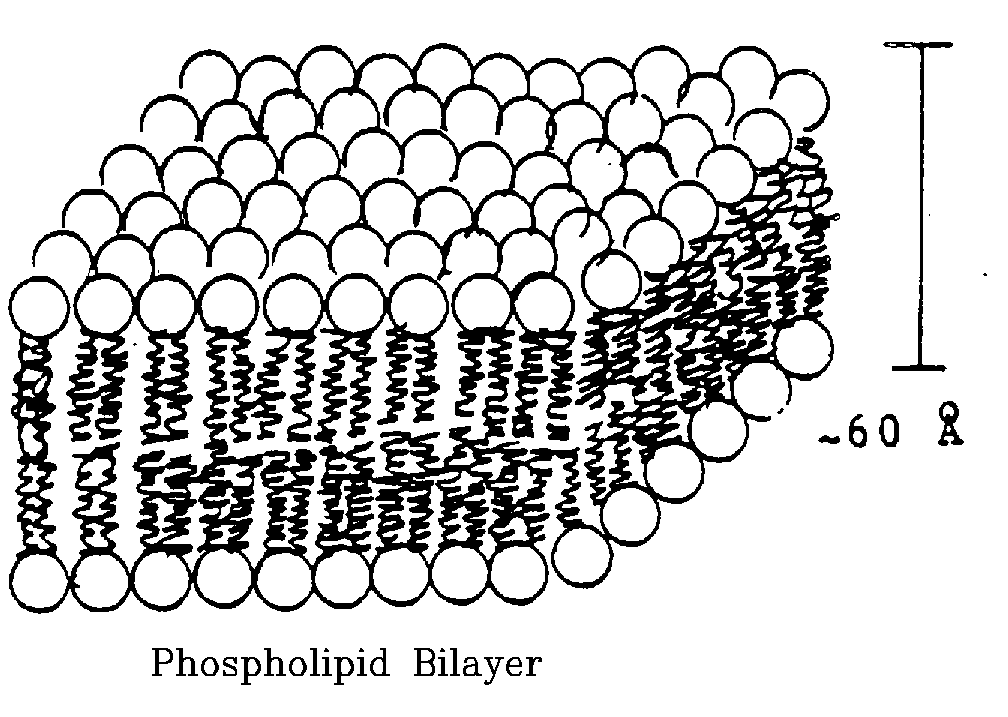
Bilayer Membrane Structure.
- Most stable configuration is with heads facing water, tails
with no water contact.
- Basic form is double layer.
- Heads always face out.
- Tails fill inside of membrane.
CHARACTERISTICS OF BILAYER MEMBRANES
- Stable yet flexible -- almost fluid.
- Especially stable surrounding small volumes like cells.
- Virtually impermeable to ions.
- Virtually impermeable to large molecules.
- The membrane acts to isolate the intracellular from the extracellular
environment.*
MECHANISMS FOR SUBSTANCES TO CROSS MEMBRANES
Diffusion through bilayer.
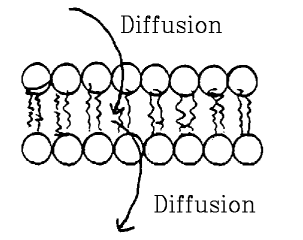
- Molecules dissolved in water must leave water, dissolve in
oil interior of membrane, then re-dissolve in water on other side.
- Movement is by diffusion.
- Membrane impermeable to substances that don't dissolve in
oil, and permeable to those that do.
- Important mechanism of permeation for:
- Gasses.
- Organic molecules like (some) drugs.
- Alcohol.
Specific membrane proteins.
- Cells regulate permeability by using specific proteins: Channels,
Carriers, Pumps. (To be discussed in detail below).
Endocytosis and Exocytosis.
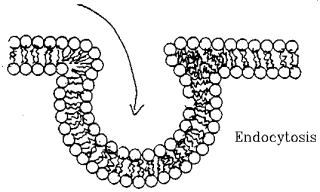
- Pouches of membrane are pinched off or expelled.
- Allows uptake or secretion of very large substances.
- Examples: Lipid uptake in gut, hormone secretion.
MEMBRANE PROTEINS
Very extensive in type and function. Functions range from immune
responses to metabolism. This section deals only with proteins
important for control of membrane permeability.
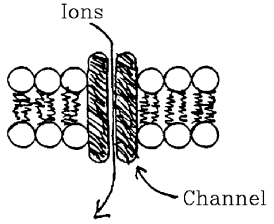
Channels.
- Large protein macro-molecules.
- Span from one side of membrane to other.
- Cylindrical hole provides pathway for ions.
- Not necessary to dissolve in oil to cross.
- Channels can selectively allow only a single kind of ion to
pass.
- Ions pass in both directions. Movement is passive -- by diffusion.
- Net movement is determined by electrochemical gradients of
bulk solutions.
- Each Channel passes hundreds of millions of ions per second,
when open.
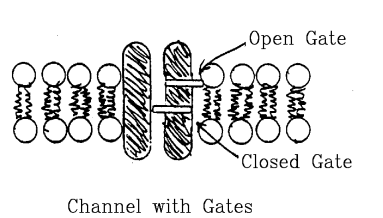
- Channels not always open (able to pass ions).
- Opening is controlled by gates.
- Gates regulated by voltage, receptors.
Channels are identified by ions that pass through them, or the
control of their gates.
Examples:
| Found in:
| Molecular Structure:
| Conduct-ance: |
Regulated by: | Permeable to:
| Blocked by: |
| Na+ Channels |
Nerve, muscle, heart | 4-fold symmetry, in a single molecule
| 15-30 pS (10-12 Siemens)
| Membrane Potential -- depolarization.
| Na+, Li+ | Tetrodo-toxin (from puffer fish)
|
| Ca++ Channels |
Nerve, secretory cells, skeletal, cardiac and smooth muscle.
| 4-fold symmetry in a single molecule.
| <1 pS | Membrane Potential -- depolarization
| Ca++, Ba++ | Verapamil, Nifedipine
|
| ACh-Activated Channels
| Skeletal muscle end plates., CNS
| 5 separate subunits. |
50 pS. | Binding of 2 ACh Molecules.
| Na+, K+, and Ca++ | Alpha-Bungaro-toxin, curare.
|
Carriers (Transporters)
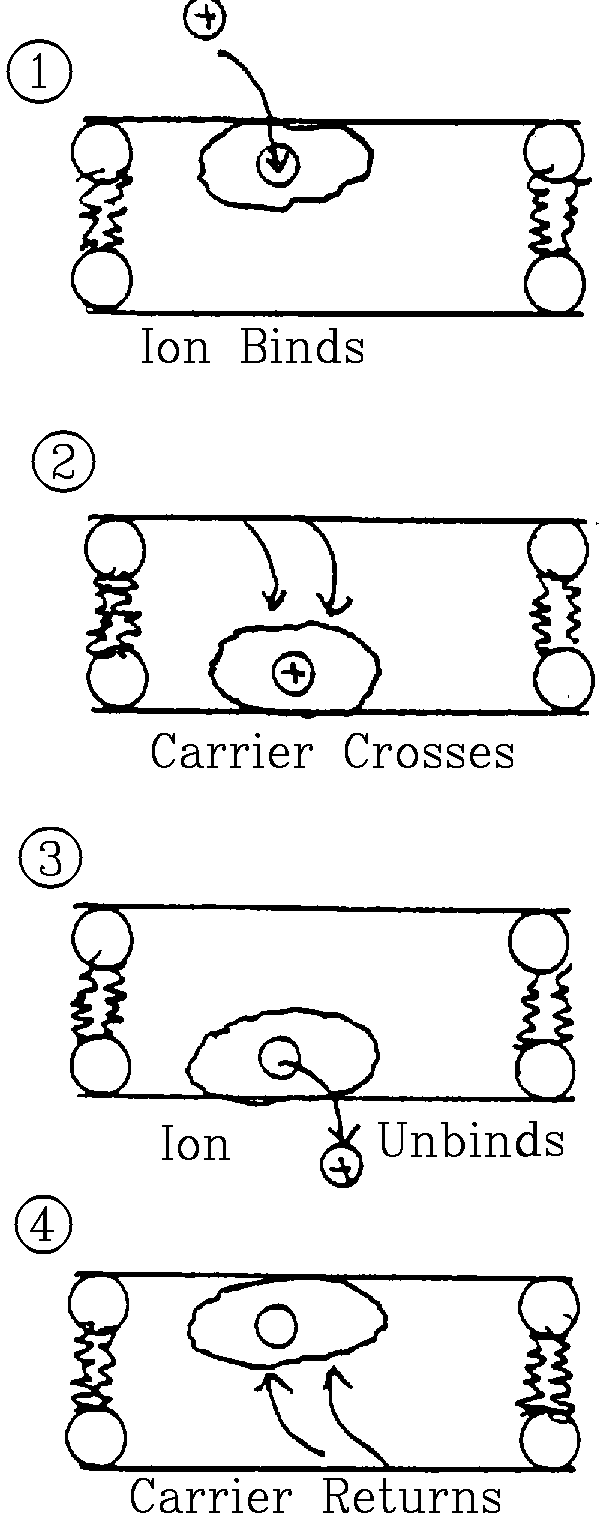
- Allow ions to cross membranes without formation of a channel.
- Mechanism involves 4 steps:
- Ion binds to protein on one side of membrane.
- Protein crosses membrane with ion bound.
- Ion unbinds on other side.
- Protein re-crosses membrane.
- Shuttle mechanism moves one (or just a few) ions each cycle.
- Maximum flux is up to 1 million times slower than for a channel.
- Transport is passive (i.e. subject to electrochemical gradient),
like channels.
- Three primary types of carrier:
Simple Carrier.
- Always carries just one ion.
- Example: Valinomycin (a natural antibiotic).
Co-transporter.
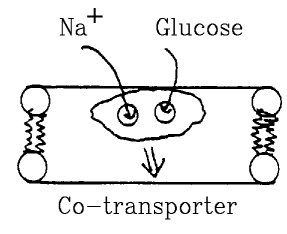
- Must bind to two different molecules before it can cross membrane.
- Gradient for one molecule can drive the other against its
gradient.
- Examples:
- Na-glucose,
- Na-amino acid transport.
- Important in kidney, digestion.
Exchange Transporter.
- Brings ion X in one direction, and must bring ion Y in the
reverse direction.
- Gradient for X can drive Y against gradient.
- Examples:
- Na-Ca exchange in heart,
- Na-H exchange in kidney,
- Cl-HCO3 exchange in blood cells.
Pumps.
- Transporters specialized for moving ions against their gradients
(uphill).
- Require metabolic energy, provided by hydrolysis of ATP to
ADP.
- Each cycle moves one or a few ions, and requires ATP.
- Most ubiquitous and important pump is Na-K-ATPase.
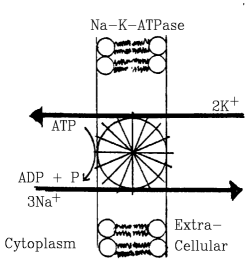
- Each cycle pumps 2 K+ ions into cell, 3 Na+ ions out.
- Requires one ATP per cycle.
- Indirectly provides energy to many other transport systems
because Na+ gradient is used for co-transport and exchange.
- Other pumps exist for Ca++ and H+ ions.
Receptors.
- Do not directly allow passage of ions.
- Act to modulate channels, carriers and pumps.
- Bind specifically to exogenous substances like transmitters,
hormones, odorants (smells) and tastes.
- When activated, modulate other membrane proteins.
- Two primary types:
- Receptors that control channel gates.
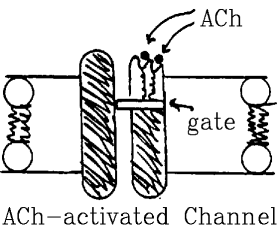
Example: ACh-activated channel, GABA-activated channel.
- Receptors that induce the production of cytoplasmic second
messengers.
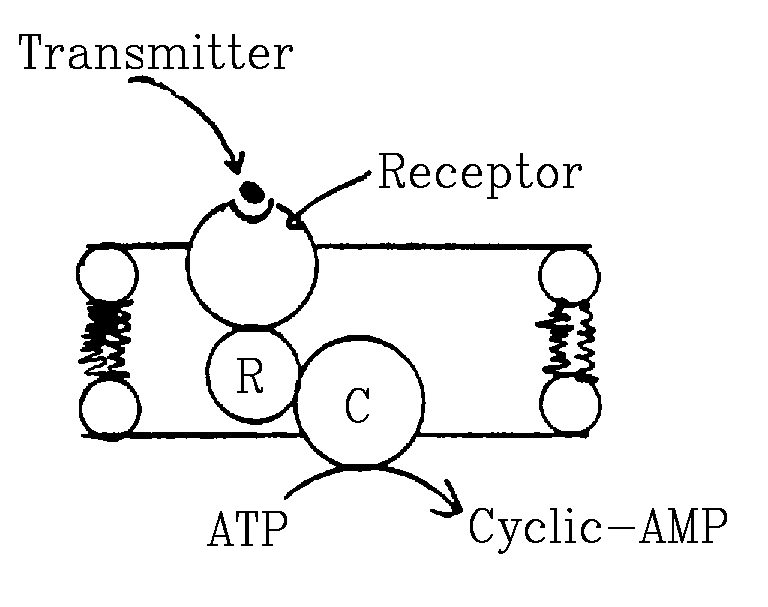
Example: Adrenergic receptors (for norepinephrine or epinephrine)
induce the production of cyclic-AMP.
- Very important for hormones and neurotransmitters.
- Frequent target of drug therapy.
- More detail to follow in other sections of course.
OVERALL STRUCTURE OF "REAL" CELL MEMBRANES
The Fluid Mosaic Model of Singer and Nicolson.
- Basic structure is phospholipid bilayer.
- Membrane proteins have hydrophobic surfaces.
- This part of proteins allows them to "float" in
oily center of membrane.
- Some proteins have hydrophilic parts that stick out into the
water on one or both sides.
- Everything is free to move in lateral direction
- Note, however -- some proteins are "tethered" and
cannot move.
- Their position is controlled by the structure of the cell.
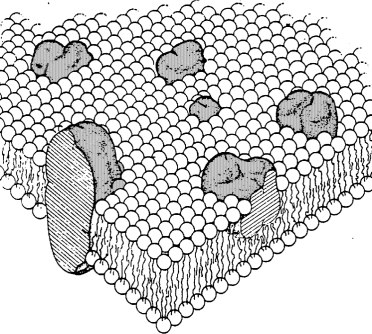
The lipid-globular protein mosiac model of Singer and Nicolson.
Schematic three-dimentional and cross-sectional views. The solid
bodies represent the integral proteins. [From S.J. Singer and
G.L. Nicolson. Science, 175:723 (1972)]










