3. Amphipathic Lipids and the Creation of Micelles.
To understand the remarkable properties of amphipathic lipids, consider the following
thought experiment.
A quantity of phospholipid is placed in a separatory funnel containing equal volumes of
olive oil and a saline solution. Then the funnel is shaken vigorously, thoroughly mixing
the initially separate (or immiscible) oil and water layers and dissolving the
phospholipid. If the layers are now allowed to separate completely once again, both the
upper, oil layer and the bottom, aqueous layer will be slightly cloudy and the interface
will be slightly more evident than it was before introducing the phospholipid. If more
phospholipid is added, and the experiment repeated, the upper and lower phases appear more
cloudy; less phospholipid produces more transparent layers. What's happening?
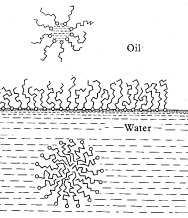 When shaken the phospholipid molecules distribute themselves between the oil
and the saline phases in an interesting manner, as illustrated to the right. Being
amphipathic, phospholipid can enter both phases but cannot completely dissolve in either!
In the saline solution, phospholipid breaks up into very small but organized structures
called spherical micelles, each of which is formed from many individual
molecules aggregating with their nonpolar tales pointing inwards and polar heads outward
(interacting with the water molecules and saline ions). As if each molecule were
"trying" to go into solution but can not! Similarily, spherical micelles are
formed in the oil phase, but the phospholipids exhibit the opposite orientation (including
trapped saline droplets at the micelle core). The micelles are large enough to scatter
light and if enough are present, each phase become cloudy, even translucent. At the
oil/water interface phospholipids readily form a different, extended structure called a planar
micelle or monolayer.
When shaken the phospholipid molecules distribute themselves between the oil
and the saline phases in an interesting manner, as illustrated to the right. Being
amphipathic, phospholipid can enter both phases but cannot completely dissolve in either!
In the saline solution, phospholipid breaks up into very small but organized structures
called spherical micelles, each of which is formed from many individual
molecules aggregating with their nonpolar tales pointing inwards and polar heads outward
(interacting with the water molecules and saline ions). As if each molecule were
"trying" to go into solution but can not! Similarily, spherical micelles are
formed in the oil phase, but the phospholipids exhibit the opposite orientation (including
trapped saline droplets at the micelle core). The micelles are large enough to scatter
light and if enough are present, each phase become cloudy, even translucent. At the
oil/water interface phospholipids readily form a different, extended structure called a planar
micelle or monolayer.
Thus, phospholipids associate spontaneously into stable micelles because of their
amphipathic properties. This particular mixing experiment simply illustrates what would
happen naturally, over a longer period of time. What stabilizes these structures?
Moreover, what do micelles have to do with plasma membranes? To answer this second
question, we next need to consider real
data from an actual experiment.
 When shaken the phospholipid molecules distribute themselves between the oil
and the saline phases in an interesting manner, as illustrated to the right. Being
amphipathic, phospholipid can enter both phases but cannot completely dissolve in either!
In the saline solution, phospholipid breaks up into very small but organized structures
called spherical micelles, each of which is formed from many individual
molecules aggregating with their nonpolar tales pointing inwards and polar heads outward
(interacting with the water molecules and saline ions). As if each molecule were
"trying" to go into solution but can not! Similarily, spherical micelles are
formed in the oil phase, but the phospholipids exhibit the opposite orientation (including
trapped saline droplets at the micelle core). The micelles are large enough to scatter
light and if enough are present, each phase become cloudy, even translucent. At the
oil/water interface phospholipids readily form a different, extended structure called a planar
micelle or monolayer.
When shaken the phospholipid molecules distribute themselves between the oil
and the saline phases in an interesting manner, as illustrated to the right. Being
amphipathic, phospholipid can enter both phases but cannot completely dissolve in either!
In the saline solution, phospholipid breaks up into very small but organized structures
called spherical micelles, each of which is formed from many individual
molecules aggregating with their nonpolar tales pointing inwards and polar heads outward
(interacting with the water molecules and saline ions). As if each molecule were
"trying" to go into solution but can not! Similarily, spherical micelles are
formed in the oil phase, but the phospholipids exhibit the opposite orientation (including
trapped saline droplets at the micelle core). The micelles are large enough to scatter
light and if enough are present, each phase become cloudy, even translucent. At the
oil/water interface phospholipids readily form a different, extended structure called a planar
micelle or monolayer.