2. The Lipid Nature of Biological Membranes
Lipids make up the bulk of biological membranes, and it's reasonable to suppose their large numbers greatly influence membrane structure and function. Indeed, it's not too far-fetched to say membrane lipids and their interactions "create" biological membranes. How do such small molecules exert such a large role? To answer this question we need to examine the structure of lipids themselves.
 Most cellular lipids are derived chemically from the three-carbon alcohol, glycerol, through covalent linkages with up to three fatty acids. Specifically, a fatty acid may form an ester with each of glycerol's three alcohol residues; the resulting lipid is called a glyceride. Thus, glycerides are a large family of molecules, each member of which contains a different combination of fatty acid (or acyl) esters. All membrane glycerides have long, non-polar fatty acid residues attached to two adjacent glycerol carbons and a polar, often charged, residue linked covalently with the third carbon. These are called diacyl glycerides or simply diglycerides. Diglycerides containing phosphate as part of the polar residue are called, not surprisingly, phospholipid. One such phospholipid - phosphatidyl choline - is illustrated on the left; double clicking on this cartoon will produce a more realistic structural image that you can manipulate in various ways using RasMol . Another major plasma membrane lipid is cholesterol, a sterol, which is illustrated below; this cartoon is also hyper-linked to a molecular model.
Most cellular lipids are derived chemically from the three-carbon alcohol, glycerol, through covalent linkages with up to three fatty acids. Specifically, a fatty acid may form an ester with each of glycerol's three alcohol residues; the resulting lipid is called a glyceride. Thus, glycerides are a large family of molecules, each member of which contains a different combination of fatty acid (or acyl) esters. All membrane glycerides have long, non-polar fatty acid residues attached to two adjacent glycerol carbons and a polar, often charged, residue linked covalently with the third carbon. These are called diacyl glycerides or simply diglycerides. Diglycerides containing phosphate as part of the polar residue are called, not surprisingly, phospholipid. One such phospholipid - phosphatidyl choline - is illustrated on the left; double clicking on this cartoon will produce a more realistic structural image that you can manipulate in various ways using RasMol . Another major plasma membrane lipid is cholesterol, a sterol, which is illustrated below; this cartoon is also hyper-linked to a molecular model.
Note in particular, that both types of molecules consist of regions that are hydrophilic, or water-loving, and hydrophobic, or water-hating. This "schizophrenic" nature of membrane lipids seems to be no accident!.
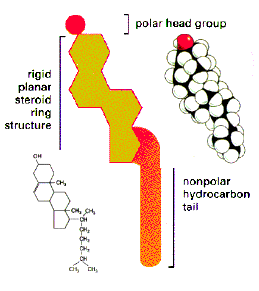 Examine the structures of common membrane lipids below and note all membrane lipids have a hydrophobic region as well as a hydrophilic one. The long hydrocarbon chains of the fatty acids (or the fatty-acid like residues) projecting to the left of each lipid do not spontaneously interact with dipolar water molecules (or readily dissolve in aqueous solutions). Conversely, the different residues projecting to the right are all polar and will readily interact with water. Thus, more technically, membrane lipids are called amphipathic molecules, because they possess distinct regions with such different affinities for oil and for water. Even the very hydrophobic and insoluble cholesterol is slightly amphipathic, by virtue of its single polar alcohol residue.
Examine the structures of common membrane lipids below and note all membrane lipids have a hydrophobic region as well as a hydrophilic one. The long hydrocarbon chains of the fatty acids (or the fatty-acid like residues) projecting to the left of each lipid do not spontaneously interact with dipolar water molecules (or readily dissolve in aqueous solutions). Conversely, the different residues projecting to the right are all polar and will readily interact with water. Thus, more technically, membrane lipids are called amphipathic molecules, because they possess distinct regions with such different affinities for oil and for water. Even the very hydrophobic and insoluble cholesterol is slightly amphipathic, by virtue of its single polar alcohol residue.
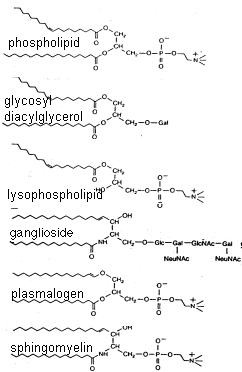 The amphipathic nature of membrane lipids contrasts strikingly with the neutral triglycerides and cholesterol esters, which are more abundant in our bodies than their amphipathic relatives but are rarely if ever found in membranes.
The amphipathic nature of membrane lipids contrasts strikingly with the neutral triglycerides and cholesterol esters, which are more abundant in our bodies than their amphipathic relatives but are rarely if ever found in membranes.
How do you think these amphipathic molecules would behave if their concentrations were increased in an aqueous environment? in an organic solvent? at the interface between an aqueous and an organic solvent? As you might imagine, their amphipathic properties cause lipids to spontaneously organize into supra- or multimolecular structures. Go on to the next page to consider the formation of lipid aggregates called micelles.
 Most cellular lipids are derived chemically from the three-carbon alcohol, glycerol, through covalent linkages with up to three fatty acids. Specifically, a fatty acid may form an ester with each of glycerol's three alcohol residues; the resulting lipid is called a glyceride. Thus, glycerides are a large family of molecules, each member of which contains a different combination of fatty acid (or acyl) esters. All membrane glycerides have long, non-polar fatty acid residues attached to two adjacent glycerol carbons and a polar, often charged, residue linked covalently with the third carbon. These are called diacyl glycerides or simply diglycerides. Diglycerides containing phosphate as part of the polar residue are called, not surprisingly, phospholipid. One such phospholipid - phosphatidyl choline - is illustrated on the left; double clicking on this cartoon will produce a more realistic structural image that you can manipulate in various ways using RasMol . Another major plasma membrane lipid is cholesterol, a sterol, which is illustrated below; this cartoon is also hyper-linked to a molecular model.
Most cellular lipids are derived chemically from the three-carbon alcohol, glycerol, through covalent linkages with up to three fatty acids. Specifically, a fatty acid may form an ester with each of glycerol's three alcohol residues; the resulting lipid is called a glyceride. Thus, glycerides are a large family of molecules, each member of which contains a different combination of fatty acid (or acyl) esters. All membrane glycerides have long, non-polar fatty acid residues attached to two adjacent glycerol carbons and a polar, often charged, residue linked covalently with the third carbon. These are called diacyl glycerides or simply diglycerides. Diglycerides containing phosphate as part of the polar residue are called, not surprisingly, phospholipid. One such phospholipid - phosphatidyl choline - is illustrated on the left; double clicking on this cartoon will produce a more realistic structural image that you can manipulate in various ways using RasMol . Another major plasma membrane lipid is cholesterol, a sterol, which is illustrated below; this cartoon is also hyper-linked to a molecular model. Examine the structures of common membrane lipids below and note all membrane lipids have a hydrophobic region as well as a hydrophilic one. The long hydrocarbon chains of the fatty acids (or the fatty-acid like residues) projecting to the left of each lipid do not spontaneously interact with dipolar water molecules (or readily dissolve in aqueous solutions). Conversely, the different residues projecting to the right are all polar and will readily interact with water. Thus, more technically, membrane lipids are called amphipathic molecules, because they possess distinct regions with such different affinities for oil and for water. Even the very hydrophobic and insoluble cholesterol is slightly amphipathic, by virtue of its single polar alcohol residue.
Examine the structures of common membrane lipids below and note all membrane lipids have a hydrophobic region as well as a hydrophilic one. The long hydrocarbon chains of the fatty acids (or the fatty-acid like residues) projecting to the left of each lipid do not spontaneously interact with dipolar water molecules (or readily dissolve in aqueous solutions). Conversely, the different residues projecting to the right are all polar and will readily interact with water. Thus, more technically, membrane lipids are called amphipathic molecules, because they possess distinct regions with such different affinities for oil and for water. Even the very hydrophobic and insoluble cholesterol is slightly amphipathic, by virtue of its single polar alcohol residue.