6a. Glycophorin - A Mammalian Erythrocyte Membrane Glycoprotein
How do the structures of known integral membrane proteins compare with the predictions made from extraction and labeling studies?
The first IMP structure to be characterized was that of Glycophorin, the major glycoprotein of mammalian RBC membranes that is responsible for M-N antigenicity. Its primary structure consists of 131 amino acids and, in addition, 16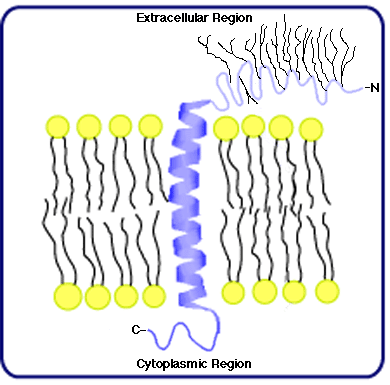 oligosaccharide branches; the peptide accounts for approximately 40% of the molecular mass, and the polar oligosaccharides provide the remaining 60% (Marchesi, et al., 1976). Labeling studies indicate the N-terminal portion of the peptide extends from the extracellular membrane surface, the C-terminal from the cytoplasmic surface, and all the oligosaccharides are attached to amino acids in the N-terminal region. (Fifteen of the oligosaccharides are o-linked (that is, attached to either serine or threonine residues in the peptide); one is n-linked (attached to asparagine).) Moreover, glycophorin's small size and the tightly clustered pattern of glycosylation suggest it spans the membrane only once, as illustrated in the cartoon to the right.
oligosaccharide branches; the peptide accounts for approximately 40% of the molecular mass, and the polar oligosaccharides provide the remaining 60% (Marchesi, et al., 1976). Labeling studies indicate the N-terminal portion of the peptide extends from the extracellular membrane surface, the C-terminal from the cytoplasmic surface, and all the oligosaccharides are attached to amino acids in the N-terminal region. (Fifteen of the oligosaccharides are o-linked (that is, attached to either serine or threonine residues in the peptide); one is n-linked (attached to asparagine).) Moreover, glycophorin's small size and the tightly clustered pattern of glycosylation suggest it spans the membrane only once, as illustrated in the cartoon to the right.
What is the secondary and tertiary structure of glycophorin? The primary structure provides a partial answer to this question. Counting from the N-terminal, the first 72 amino acids are mostly polar in nature, as are those found at the C-terminal (from amino acid 93 to amino acid 131). A sequence of 20 apolar or hydrophobic amino acids exists between position 73 and 92, however, and it's reasonable to hypothesize this region links the much more polar N- and C- terminal regions that project, respectively, into the extracellular environment and into the cytoplasm. These apolar amino acids likely generate a secondary structure known as an alpha-helix; moreover, a helix consisting of 20 amino acids just about spans the membrane once. These features are consistent with the single-pass model of glycophorin illustrated above.
Is this reasonable model structurally accurate? Certainly, it's consistent with all the circumstantial evidence provided by labeling and model building, but is the model also supported by direct structural studies? Proceed to the next page to examine a spectroscopic analysis of glycophorin structure.
 oligosaccharide branches; the peptide accounts for approximately 40% of the molecular mass, and the polar oligosaccharides provide the remaining 60% (Marchesi, et al., 1976). Labeling studies indicate the N-terminal portion of the peptide extends from the extracellular membrane surface, the C-terminal from the cytoplasmic surface, and all the oligosaccharides are attached to amino acids in the N-terminal region. (Fifteen of the oligosaccharides are o-linked (that is, attached to either serine or threonine residues in the peptide); one is n-linked (attached to asparagine).) Moreover, glycophorin's small size and the tightly clustered pattern of glycosylation suggest it spans the membrane only once, as illustrated in the cartoon to the right.
oligosaccharide branches; the peptide accounts for approximately 40% of the molecular mass, and the polar oligosaccharides provide the remaining 60% (Marchesi, et al., 1976). Labeling studies indicate the N-terminal portion of the peptide extends from the extracellular membrane surface, the C-terminal from the cytoplasmic surface, and all the oligosaccharides are attached to amino acids in the N-terminal region. (Fifteen of the oligosaccharides are o-linked (that is, attached to either serine or threonine residues in the peptide); one is n-linked (attached to asparagine).) Moreover, glycophorin's small size and the tightly clustered pattern of glycosylation suggest it spans the membrane only once, as illustrated in the cartoon to the right.