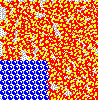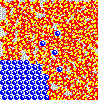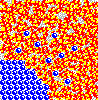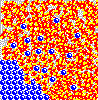

Although not obvious here, remember the solute particles that dissolve into solution are always closely associated with a shell of water molecules. The water in this shell is not tightly attached, however, and it exchanges with other water continuously as if in a solvent "tag team". Also, solute molecules may associate with one another as well, creating transiently larger particles.
These associations - of solute with solvent and of solute with itself - reduce the number of free particles and their overall chemical activity in solution. When solute associates with itself, its activity decreases. When water forms "hydration shells" around solute, its activity also goes down. Thus, the more concentrated a solution - the higher the amount of solute it contains - the lower the water activity.
Look at the next page to see this effect of solute on water in "action".