2. (28
pts) While you're enjoying the “intense” Spring sunshine in Vermont, I thought
you might like thinking about some interesting data generated by the use of
polarized laser energy, another very intense form of illumination. I've modified the problem from one that
appears in The Problem Book (which
accompanies Molecular Biology of the
Cell by Alberts, et al).
The movement of single motor proteins
can be monitored using polarized laser light.
Such illumination creates a circular interference pattern with a
circular force field that ranges from zero at the center to a few piconewtons
at the periphery (about 200 nm from the center). Individual molecules entering this interference pattern are
pushed towards the center and "captured" there, unless they possess
sufficient kinetic energy to escape.
These interference patterns are often called "optical
tweezers" because scientists can use them to move molecules about, simply
by repositioning the interference patterns. And the actual work accomplished by
motility mechanisms can be estimated from the force necessary to escape such
constraints. Nifty!!
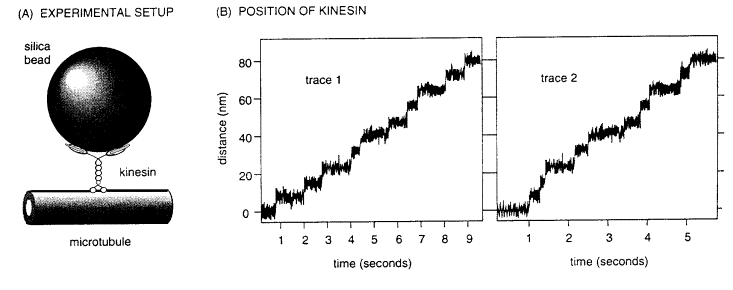
One experiment used optical tweezers
to position a kinesin molecule on a microtuble attached to a coverslip (as
illustrated below in Fig. A.). The
behavior of the kinesin could not be resolved microscopically but its movement
was readily tracked by attaching a much larger silica bead to it. When incubated in an appropriate
physiological solution, the bead vibrated with the kinesin molecule, due to
thermal kinetics, but it did not move away from the center of the interference
pattern. When ATP was added to this
test set-up, the bead began to migrate away from the center and towards the one
end of the microtubule.
As it moved along the microtubule,
kinesin encountered the force of the interference pattern, which is thought to
simulate the kind of loads born by kinesin in moving vesicles around the
cell. Traces of two kinesin molecules
along a microtubule are illustrated in Fig. B.
Consider these data and answer all
the following questions.
1.
(4 pts) What supplies the free energy needed for unidirectional (non-Brownian)
movement along the microtubule?
2. (6 pts) What is the average rate of kinesin
movement and what is the average step length?
3. (8 pts) In each
protofilament the tubulin subunits repeat at 8 nm intervals. Given the average step length and this
repeat interval, postulate a detailed mechanism for the movement of kinesin.
4. ( 4 pts) How many ATP molecules are likely hydrolyzed per
step? How did you arrive at your
answer?
5. (8 pts) Towards which end of
the microtubule does kinesin move, and what biochemical constraints might
polarize this movement? (Recall that
dynein moves along MT in the opposite direction.)