1.
Insects lack red blood cells. Instead,
an oxygen carrier/protein very similar to hemoglobin is secreted directly
into insect blood. Briefly hypothesize how the synthesis of this
protein differs from that of its vertebrate relative and describe one direct
test of your hypothesis and its possible results.
A. What does the question ask?
Insects lack red blood cells. Instead,
an oxygen carrier/protein very similar to hemoglobin is secreted directly
into insect blood . . .
The first two sentences of the question asks you to think about the synthesis of two similar proteins, one free in the blood (insects) and one contained within a red blood cell (vertebrate). While implicitly stated in the question, basic knowledge of red blood cell structure and function (from lab or lecture) is necessary to know that vertebrate hemoglobin is contained in erythrocytes:
Briefly hypothesize how the synthesis of this protein differs from that of its vertebrate relatives . . .
The question then asks you to trace the path of the insect oxygen carrier/protein during its synthesis and compare the path to hemoglobin synthesis (not secreted).
. . .and describe one direct test of your hypothesis and its possible
results.
Finally, you are asked to design an experiment to test your hypothetical pathway. Furthermore, the question asks to discuss possible results. Thus, answering this question completely requires that:
1. You recall how secreted and non-secreted proteins are synthesized,
2. You formulate a hypothesis with regards to the insect oxygen carrier, a secreted
protein,
3. You briefly describe a test, and
4. You describe possible results of the test.
All four parts are crucial to an excellent answer.
Proposing an experiment is not enough; you must discuss possible results! As long as the proposed experiment is logically based on a rational answer to the previous parts of the question, credit will be awarded even though your "facts" may be partially or completely wrong. Several experiments based on readings, lecture and lab notes, as well as your own logical creativity are possible.
2. What
question is NOT being asked?
No functional information about hemoglobin or oxygen carrier/protein is required to answer the question. Also it might be tempting to include information about the osmotic behavior of red blood cells because one lab was dedicated to the subject, but such information is not relevant to the question and should be omitted. You may be penalized for including extraneous material but more importantly doing so wastes your time.
3. What's
ambiguous about the question?
If anything doesn't make sense to you, ask the professor specific pointed questions. Don't say, "I don't understand the question." instead ask "What do you mean by __________?" or "Could you clarify __________?"
4. Now
answer the question before proceeding further.
QUESTION: Insects
lack red blood cells. Instead, an
oxygen carrier/protein very similar to hemoglobin is secreted directly into
insect blood. Briefly hypothesize
how the synthesis of this protein differs from its vertebrate relative and
describe one direct test of your hypothesis and its possible results.
Sample Responses: Answer #1
|
Overview: He begins
the question by reminding himself that hemoglobin is within red blood
cells while insect protein is secreted.
Based on this fact, he traces the path of synthesis for a secreted
peptide and compares it with hemoglobin's synthesis. He concludes that the oxygen carrier is secreted
because it has a signal sequence (and possible targeting sequences)
which hemoglobin lacks. He proposed
to test for the presence of a signal sequence on the oxygen carrier
by analyzing mRNA. The oxygen
carrier is expected to have a hydrophobid end. |
ANSWER
COMMENTARY
|
Because
the insect protein is secreted directly into the blood, it will be co-translationally
imported in the ER and targeted for the secretory vesicles.
Hemoglobin, on the other hand, is a product of red bloods cells,
and remains within these cells. Therefore, the insect protein will, when
it is first translated, begin with a hydrophobic signal sequence to
allow it to enter the ER. (It
may also have a second signal added to it in the ER or Golgi to target
it to the secretory vesicles.) We
might be able to test the idea that the insect protein will begin with
a signal sequence while the hemoglobin will not by isolating the genes
for each in vitro and analyzing their products.
We expect the oxygen carriers produced by insects to have a hydrophobic
end and hemoglobin not to. |
The
synthesis pathways are clearly and correctly traced in the first paragraph.
The alternating structure of the 1st 2 sentences is
effective especially for answering compare/contrast questions. The
hypothesis is basically that the insect carrier protein is secreted
because it contains a signal sequence.
Hemoglobin does not contain such a sequence, so he designed an
experiment to detect the sequence. A
good test, but unfortunately he doesn't specifically state how he intends
to do so experimentally. He
could have referred to specific experimental techniques learned in lecture. In a "brief" answer it is not necessary
to provide technical details; nevertheless, more information is required
than provided here. The
results he would expect to generate from the experiment are not well described. The results need to be more explicitly related
to the hypothesis: 1) hydrophobic end=signal sequence, 2) signal sequence=secretion
and 3) secretion=protein directly in the blood. |
A
Second Example:
|
Overview: First, the
student concludes the syntheses of hemoglobin and the insect oxygen
carrier must be different because the fates of the two proteins are
different. Then, she proceeds
to detail how the oxygen carrier is synthesized, processed, and secreted
using text and diagrams. As
a logical extension, she decides to experimentally monitor the synthetic
pathway of the two proteins by a pulse-chase experiment.
The results are presented in a graph and conclusions are drawn
from the graphs to explain the differences between the two proteins. |
ANSWER
COMMENTARY
|
Hemoglobin
in RBC is synthesized to stay inside the RBC while the oxygen carrier/protein
in insects is synthesized to be exported.
As a result, processes of synthesis is different. The hemoglobin is synthesized in the cytoplasm
and stays in cytoplasm therefore it lacks a signal peptide. The oxygen carrier/protein will be exported
so it will have a signal protein that will associate with a stop particle
that will halt synthesis until it comes in contact with rER docking
site and translation will resume. The
signal peptide will be cleaved with signal peptidase. Then the protein will be brought to the cis-side
of the Golgi via transitory vesicles where it will be further processed.
The protein probably binds to a receptor side on the trans side
of the Golgi and a secretory vesicle is formed with the protein inside.
The secretory vesicle moves towards the plasma membrane where
it fuses with the membrane, the protein is released into the insect
blood and the secretory vesicle membrane becomes part of the plasma
membrane. Diagrams: |
The answer is laid out effectively by starting
with general knowledge. In doing
so, the reader is told what to expect from the rest of the answer; a
more detailed comparison of hemoglobin and oxygen carrier pathways. She traces the pathway of the oxygen carrier
protein synthesis from the rER-->Golgi-->secretory-->plasma
membrane-->insect blood. Some
of the details are fuzzy – e.g., “stop particle” instead of SRP, and
it’s not clear what “further processed” means.
Otherwise, a Very Good answer! Note how helpful, but how simple, a diagram,
is for organizing and expressing thoughts. |

Continuing Example 2:
|
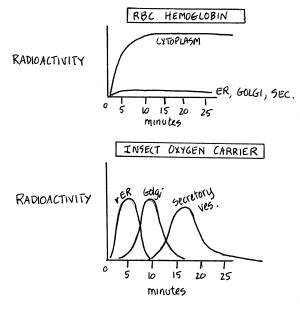
To
observe the movement of the hemoglobin and oxygen carrier/ protein,
one could perform a "pulse-chase experiment." Radioactively labeled nucleotides would be added for five minutes
and then cells would be incubated with non-labeled nucleotides for 30
minutes and measure where the radioactivity is at certain times. The result probably would show:
Therefore,
hemoglobin stays inside the cytoplasm of the cell, and has no signal
sequence. The insect oxygen
carrier/protein goes through the rER, Golgi, secretory vesicles and
is exported and has a signal peptide that allows it into the rER. |
An excellent test for this hypothesis,
but one requiring radioactive amino acids and not nucleotides! Graphs are effective ways of communicating
data, and in this example they are especially effective for comparing
the different pathways. The exact shapes of the curves are imprecise, for idiosyncratic reasons beyond
the scope of BI250 (involving protein turnover, for example). Furthermore, the data are interpreted correctly
and related to the presence of a signal sequence and the fates of newly
synthesized proteins, either inside or outside the cell. Note also that what is being tested -- the
pathway -- is quite different than what was tested in the first Example. Given the contexts, both are reasonable tests
and equally correct answers! An Excellent answer! |
Now that you've answered the question and examined two responses in detail, review the question and critique the following 2 responses yourself in the space provided on the left.
ANSWER #3
COMMENTARY
|
Because the insects' oxygen carrier/protein
is a secretory protein, its synthesis will initially differ in that
it will produce a hydrophobic signal sequence. This sequence will then be bound by SRP (signal
recognition particle) until the ribosome migrates to the rER. Docking protein in the rER membrane will
then recognize SRT which in turn activates TRAM, forming a channel in
the rER membrane through which the nascent polypeptide may then pass. The hemoglobin of vertebrates, by contrast,
will likely be synthesized in the cytoplasm and contain no such signal
sequence for import into the rER because it is a cytoplasmic protein. A test of this hypothesis might be to isolate
the gene for the insect oxygen carrier and delete the portion which
codes for the signal sequence. This
would presumably inhibit the entry of the polypeptide into the rER and
thus it would not be secreted. |
|
Your critique might usefully lead you to edit the example, making it more accurate in the process. Use the space below for your edition –
|
|
ANSWER #4 |
COMMENTARY |
|
|
An obvious
difference is that the insect protein is secreted while hemoglobin is
retained within the red blood cell.
Thus, the protein (insect) at one point probably had a signal
sequence on the amino end that enabled the protein to enter the lumin
of the rER. Hemoglobin lacked this type of signal sequence
and thus moved into the cytoplasm rather than into the rER. Once this insect protein went into the rER, it possibly went through
some modifications such as losing its signal sequence and/or glycosylation.
Hemoglobin had no signal sequence to lose and would not go through
glycosylation. Modification such as sulfur bridges may occur
with both. In fact, it does
with hemoglobin as it gains its quaternary structure. The insect may lack quaternary structure,
we cannot tell. However, any
tertiary or quaternary structure will take place here. The hemoglobin does not leave the cytoplasm but the insect protein moves
on through vesicles to the Golgi. Here
glycosylation will finish up as will other modifications. From here it moves to secretory vesicles
and is secreted. The hemoglobin
remains within the RBC. A way to test if the difference of location of modification does exist
between the proteins, one could get some antibodies to the protein and
inject them into appropriate cells.
These antibodies would cling on to the protein thus locating
them. Most probably, the insect protein would be found in the rER, the Golgi,
or vesicles going toward the Golgi.
Secretory vesicles is another possibility. Hemoglobin will be found in the cytoplasm. If these results were found, the difference
exists. If one wanted to get more specific (i.e. test for glycosylation) one
could take both proteins and stain them with dyes that stain positive
for glycoproteins. It is possible
that the insect protein may contain oligosaccharides. It is very doubtful that hemoglobin would. |
|||
2. Remember this cartoon of a biological membrane? Suppose the cartoon represented a plasma membrane, consider the ways in which the various proteins are organized in association with the membrane and answer the following questions:

A. (15 points) Compare and
contrast the likely biosynthetic pathways of proteins numbered 2, 5 and 6.
Diagrams would help!
B. (8 points) Once completely synthesized, how are proteins numbered 1 and 6 likely to be delivered accurately to their appropriate sites of function? What are the targeting mechanisms?
C. (5 pts) How would you test your hypothesis concerning either targeting mechanism?
D. (6 pts) Suppose the membrane illustrated were part of a lysosomal vesicle, how would your answers to A. and B. above differ, if at all?
2.A. Here’s a more interesting and difficult version of the same question, using data instead of a cartoon to provide useful information:
The cDNA of a gene coding for an interesting membrane protein has been cloned, amplified and sequenced, and the amino acid sequence of its probable translation product inferred from the nucleotide sequence. The protein contains 240 amino acids and exhibits an apparent molecular weight in SDS-PAGE of approximately 30 kDa. A hydropathy plot of the protein’s primary structure is presented in the figure below, numbering the amino acids from the amino terminal end to the carboxy terminal as is customary (designated "N" and "C" respectively).
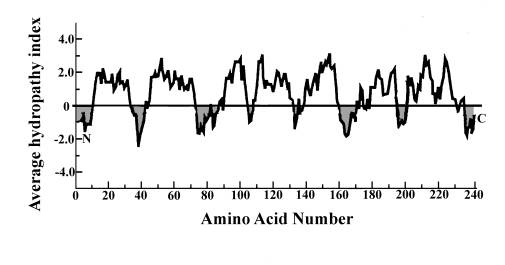
A.
(4 pts) Assuming the protein is an
IMP, how many times does it span the membrane?
B.
(8 pts) How does this association with the membrane come about? Describe its synthesis.
C.
(4 pts) If the protein were to be
glycosylated during its biosynthesis, indicate with arrows which regions might
be glycosylated.
D.
( 8 pts) If the protein were to permanently reside (and function) in the ER,
how might its biosynthetic pathway differ from that taken by a plasma membrane
IMP. How might this difference in
targeting be effected? Diagrams would
help your description.
E.
(6 pts) How might you test your hypothetical targeting mechanism for this
protein? What would the results show?
3. (26 pts) Consider the synthesis of insulin, a protein hormone with molecular weight of approximately 5.8 kDa which is secreted by endocrine cells of the pancreas in response to elevated blood glucose levels. The functional hormone consists of two subunits, “A” with a molecular weight of 2.3 kDa and “B” with a molecular weight of 3.5 kDa, linked by disulfhydryl bridges; the tertiary structure of the A chain is also maintained by an intramolecular disulhydryl linkage. The primary amino acid sequence of the subunits and the location of the disulfhydryl bridges is illustrated in the figure below.
Reduction of any of the three disulhydryl linkages inactivates the hormone; neither subunit by itself exhibits any insulin activity.
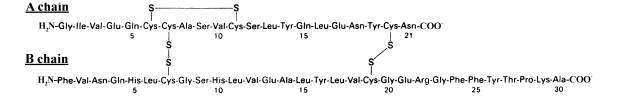
To examine the synthesis of insulin, homogenates of the endocrine gland were fractionated into organelle components and examined by SDS-PAGE (under oxydizing conditions that preserved the disulfhydryl linkages). The presence of insulin in each of these fractions was then determined using a radioactively labeled antibody specific for the hormone. An autoradiogram of the gel is reproduced below: each band represents the binding of the labeled antibody and a band’s molecular weight is indicated in the right hand margin. (The technique is called a “Western” and the additional procedural details are irrelevant here.) Consider these data and what you know about protein synthesis, and answer all the following questions.

A. (4 pts) Insulin is not a glycoprotein, although other Golgi-processed proteins produced by these glands are. How come?
B. (4 pts) Based on these data, is insulin the product of 1 or 2 genes? Why?
C. (8 pts) Describe the synthesis of insulin, using a diagram, that is consistent with these data and with your current understanding of protein synthesis.
D. (5 pts) Suggest an additional experiment that would test your hypothesized biosynthesis pathway and describe clearly what the results would indicate.
E. (5 pts) If insulin mRNA were translated in a test-tube, using a vesicle-free, SRP-free but otherwise complete cell-free translational system, how many different product(s) would be detected using the antibody described above. What would be its (their) molecular weights? Why?
Here’s a different kind of question!
Answering it requires you to read and understand two Abstracts of research
presented at an annual meeting of the American Society for Cell Biology.
By their very nature, such Abstracts are written in a terse, almost
skeletal fashion and their comprehension requires the reader to make use of
background information he or she has already mastered (and to read the Abstracts
carefully).
4. (30 pts) Much interest (and controversy) has developed in the past two decades concerning the "turnover" of the plasma membrane. How is this organelle synthesized, how is it degraded, and how are these two processes maintained in a steady-state balance? Our understanding of this phenomenon has been complicated by the observations that turnover may itself be part of such other important cellular processes as secretion (exocytosis) and both "feeding" and signalling (endocytosis). Consider this complex phenomenon in the light of what you have learned so far this term and the 2 sets of results abstracted below of work done in the same laboratory, and answer the following questions.
Binding and Internalization of GnRH by Rat Pituitary Gonadotrophs. T.M. Duello, T.M. Nett* and M.G. Farquahar, Section of Cell Biology, Yale University School of Medicine, New Haven, CT and Department of Physiology and Biophysics, Colorado State University, Fort Collins, CO.
Gonadotropin-releasing hormone (GnRH), like other peptide hormones, exerts its initial action in regulating secretion of gonadotropins (LH and FSH) by binding to specific plasma membrane receptors on the surface of the pituitary cells that secrete the LH and FSH. These cells are called gonadotrophs. Studies using fluorescent derivatives and ferritin conjugates of GnRH analogues have demonstrated their initial, diffuse binding to the cell surface followed by formation of aggregates which are gradually internalized by endocytosis. In this in vivo study we followed the fate of bound hormone by EM autoradiography using an 125I-labeled analogue of GnRH. This analog was previously shown (Mol. Cell. Endocrinol. 19: 601, 1980) to be taken up to a greater extent (~ 3-fold) and retained longer (~ 4-fold) by gonadotrophs than the native hormone. Ovariectomized rats were given intracarotid injections of 20 ng of the labeled analogue, and the pituitaries were fixed at 5, 15, 30, and 60 min. thereafter. A 1 min. pretreatment with a 200-fold excess of unlabeled analogue blocked binding at the 15, 30, and 60 min. intervals as demonstrated by very low cpm/pituitary gland and the absence of autoradiographic grains over gonadotrophs. The major findings were as follows: 1) At early time points ~50% of the grains were found within two half distances of the plasma membrane, the grain density decreasing over time; 2) the labeling of lysosomes was initially low and increased dramatically by 60 min.; 3) the grain density over the nucleus (<0.6), Golgi elements (<1.2), and rough ER (<1.2) remained relatively low at all time points; and 4) grain density of secretory vesicles was high (2.2-4.8) at all time points.
And -
Iodinated Cell Membrane Components Are Transported to the GolgiComplex. P. Wilson*, D. Sharkey*, N. Haynes*, P. Courtoy* and M.G. Farquhar. Section of Cell Biology, Yale University School of Medicine, New Haven, CT.
Recently it was shown that ferritin bound electrostatically to the plasma membrane of IgG-secreting murine myeloma cells is internalized by endocytosis and appears in the stacked Golgi cisternae and secretory vesicles after 60 min. (Ottosen et al, J. Exp. Med. 152:1, 1980). In this study we covalently labeled plasma membrane components of cultured myeloma cells using the lactoperoxidase iodination (125I) procedure of Hubbard and Cohn and followed the subsequent fate of labeled components by EM autoradiography. Characterization of the iodinated species revealed that ~15% of the label was incorporated into lipids and ~85% into proteins. Cellular incorporation of 125I was a linear function of [125I] in the medium. In a separate experiment it was shown that iodination did not affect significantly the rate of release of 14C-labeled IgG (the normal secretory product of the myeloma). In 3 experiments EM autoradiographic analysis of cells fixed immediately after iodination at 4oC showed up to ~80% of the total grains to be within two half distances (HD) of the plasma membrane and only 2% over the Golgi region. After 1 hour at 37oC, 50-60% of the label remained associated with the plasma membrane and up to 23% was found in the Golgi region. Thus, 25-50% of the total internalized label was found in the Golgi region. Of the latter, the majority of the grains were found either directly over or within 2HD of the Golgi cisternae and secretory vesicles. At no time were > 1% of the total grains found over lysosomes. (Supported by USPHS Grant AM 17780.)
1. (8 pts) How are the fates of the plasma membrane in A. and B. being studied? Briefly describe each method and the component(s) of the plasma membrane that is (are) affected. Would you expect the methods would measure identical aspects of plasma membrane turnover? Why or why not?
2. (8 pts) Describe briefly the results and the significance of each experiment
taken individually.
3. (8 pts) Discuss critically how both sets of results contribute to our understanding of plasma membrane turnover and of possible functions of the Golgi complex.
4. (6 pts) Describe one additional experiment that would provide useful information and describe clearly what the results would show.
Here’s a longer, less open-ended and more complex (and wordier)
version of the same question (used for a take-home Final Exam!).
Work your way through the data and questions and see if you come away
with a similar understanding of plasma membrane recycling.
4.a. (45 pts) Cells and their organelles are dynamic entities. In particular, cellular membranes seem constantly in motion, carrying out their various functions and being newly synthesized, degraded, recycled or transformed. The existence of these dynamic phenomena is well-document, but we do not yet completely understand how the various processes are regulated.
For example, under some circumstances a portion of the plasma membrane (PM) may be endocytosed and become associated with lysosomal vesicles. In another context, labeled PM becomes associated with different, non-lysosomal organelles following endocytosis. (See data from the previous question, reproduced in more detail in i. And ii. Below.) Data from more recent research, carried out in a laboratory near the one that produced the data in i. And ii., are presented in iii. below. You may assume all three data sets were obtained under similar conditions unless described differently.
I. Many cells that secrete hormones are themselves under hormonal control. One such cell, the pituitary gonadotroph, secretes two polypeptide hormones (LH and FSH) when stimulated by GnRH (Gonadotropin-releasing Hormone). To investigate the regulation of LH and FSH secretion, a biologically active, radioactively iodinated (125I) analogue of GnRH was injected into rats. At varying periods of time post injection, pituitary glands were fixed and sectioned, and the sections were then examined by EM-autoradiography. Photographic grains resulting from radioactive decay were counted and their location in the sections noted. These data are presented in the table below, as the percentage of total grains per cell.
|
|
Time Post Injection, min |
|||
Location |
5 |
15 |
30 |
60 |
|
1. Plasma
Membrane |
60% |
15% |
20% |
5% |
|
2. AP’ase-positive
vesicles |
20 |
30 |
60 |
80 |
|
3. GalTran’ase-positive
vesicles |
2 |
2 |
2 |
2 |
|
4. rER |
3 |
3 |
2 |
2 |
|
5. Nucleus |
1 |
1 |
1 |
1 |
|
6. Secretory Vesicles |
5 |
8 |
9 |
8 |
Note: “AP’as- positive” indicates the presence of acid phosphatase;
“GalTran’ase-positive” indicates the
presence of galactosyl transferase.
II. In another set of experiments using a culture of cancerous bone-marrow cells (myeloma), an iodination procedure was used to label plasma membranes directly, using 125I. The fate of the radioactive label was then followed with EM-autoradiography. Chemical analysis of the iodinated species revealed 15% of the label was covalently bound to membrane lipid and 85% to IMP. (Separate experiments confirmed the labeling process did not affect the viability of these cells or their ability to secrete IgG.) Cells fixed 5 and 60 min after labeling showed the following distribution of grains.
|
|
Time Post
Labeling, min |
|||
Location |
5 |
60 |
|
|
|
1. Plasma Membrane |
85% |
55% |
|
|
|
2. AP’ase-positive
vesicles |
2 |
2 |
|
|
|
3. GalTran’ase-positive
vesicles |
2 |
25 |
|
|
|
4. rER |
1 |
1 |
|
|
|
5. Nucleus |
1 |
1 |
|
|
|
6. Secretory Vesicles |
2 |
10 |
|
|
III. The internalization and intracellular transport of the FC receptor (FCR was examined in cultures of mouse macrophages using 125I-labeled antibodies against the receptor. Following a brief pulse for labeling the receptors, labeled antibodies remaining at the surface were removed with a brief wash at pH 4.0, cells were incubated for varying periods of time and the intracellular fates of the label was monitored by cell fractionation and radioisotopic assay. The data in Table I was obtained using antibody fragments (F’ab) with only one receptor binding domain; those in Table II made use of intact IgG (with two receptor binding domains).
Table I
|
|
Time Post Washing, min |
|||
Location |
5 |
15 |
30 |
60 |
|
1. Plasma Membrane |
10% |
40% |
50% |
60% |
|
2. Lysosomes |
8 |
9 |
7 |
7 |
|
3. Trans-Golgi vesicles |
3 |
4 |
3 |
5 |
|
4. Cis-Golgi vesicles |
4 |
5 |
5 |
4 |
|
5. rER |
1 |
1 |
1 |
1 |
|
6. Nucleus |
2 |
1 |
2 |
1 |
|
7. Secretory Vesicles |
62 |
40 |
32 |
22 |
Table II
|
|
Time Post Washing, min |
|||
Location |
5 |
15 |
30 |
60 |
|
1. Plasma Membrane |
12% |
8% |
7% |
7% |
|
2. Lysosomes |
60 |
60 |
50 |
50 |
|
3. Trans-Golgi
vesicles |
5 |
10 |
12 |
11 |
|
4. Cis-Golgi
vesicles |
4 |
5 |
5 |
4 |
|
5. rER |
1 |
1 |
1 |
1 |
|
6. Nucleus |
1 |
2 |
1 |
1 |
|
7. Secretory Vesicles |
18 |
17 |
25 |
28 |
A. (25 pts) Discuss critically and as completely as possible what these data indicate concerning the regulation of plasma membrane flow/turnover during brief periods in the lives of cells. Illustrate your discussion with well-labeled diagrams and attend closely to the following questions:
i. what components in each experiment are being labeled and traced?
ii. what appear to be the fate of these components?
iii. how do these results compare with other endocytotic experiments you have
examined: e.g., LDL uptake by fibroblasts?
iv. are the date equivocal – that is, open to different interpretations? How so?
v. what additional experiment could be performed to remove ambiguity or to test your
hypothesis concerning plasma membrane turnover?
B. (10 pts) The data in all experiments represent relative amounts of label in each fraction at the indicated time points. Considering both parts of Experiment III, would you expect the total amount of label within the cell to vary among the time periods. Why or why not?
C. (10 pts) Given your answer in C. above, hypothesize how the insertion of newly synthesized FC R into the plasma membrane might occur and how the insertion might be regulated.
5. (26 pts) The cells lining the digestive system of cnidarians (hydra, jellyfish, corals and their relatives) directly ingest and then internally digest unicellular prey. Curiously, some cnidaria (or coelenterata as they used to be called) have evolved mechanisms for forming a commensal relationship with the single cell alga, Chlorella (ingesting but not digesting them). Speculate how such a relationship might work at the cellular level, consider the following, slightly edited Abstract of research presented at an annual meeting of the American Society for Cell Biology, and answer the following questions.
Endosybiont Perturbation of Phagosome Digestion. Thomas C. Hohman, Paul L. McNeil, and Leonard Muscatine. Department of Biology, UCLA, Los Angeles California.
Unicellular Chlorella are capable of living with the digestive cells of the fresh water colenterate Hydra viridis. These symbiotic algae are phagocytized by hydra digestive cells but avoid host digestion and persist at constant numbers within host cells. In contrast, heat-killed symbionts are rapidly degraded and do not persist within host cells. Acid phosphatase activity could not be detected in phagosomes containing live symbionts, whereas this enzyme was found in over 50% of the vacuoles containing heat-killed symbionts one hour after phagocytosis. Darkness and DCMU [an herbicide], both of which inhibit photosynthesis and hence the release of photosynthetic produces, also abolish persistence. Neither of these latter treatments affected symbiont viability. Symbiotic algae are not only recognized by digestive cells as particles not to be attacked, they are also selected for transport within host cells from the apical site of ingestion to a basal position of permanent residence. This process too is disrupted by DCMU and darkness. Algal persistence and transport may both, therefore, be signaled by the release of photosynthetic products.
A. (8 pts) What is the usual fate of material that enters a cell, say a macrophage, by phagocytosis? Describe these events with the aid of a diagram.
B. (10 pts) Based on the Abstract how might Chlorella escape the usual fate of phagocytized material? Your hypothesis must be as specific and as detailed as possible.
C. (6 Pts) Briefly discuss at least 1 critical test of your hypothesis and indicate clearly how the results would prove or disprove it.