Preface | Introduction to Problem Solving | Problem Sets | Acknowledgments
| A.1 RBC Organization Next Problem>> | PPrint PDF |
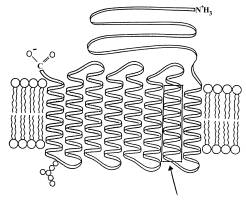 |
a. (10 pts.) Consider the lipid molecules illustrated, identify their
parts and briefly describe how their organization is stabilized.
b. (5 pts.) Indicate clearly which side of the membrane is exterior and
which side faces the cytoplasm, and briefly defend your designation.
As a strategy for answering these questions, first ask yourself two additional
questions:
1. What do the questions ask?
Question A. asks you to first identify each part of the lipid molecules
in the diagram, referring specifically to them in the figure.
Then, explain how the overall lipid organization is stabilized.
Note the two-part nature of the question: do the identifications in the
first part aid your mechanistic explanation of their organization in the
second part?
Question B. simply asks you to identify the exterior and interior of
the membrane in the diagram, and you must provide a specific rationale
for your conclusion.
2. What questions are NOT being asked?
Neither question requires any functional or experimental information about red blood cells, to be answered correctly.
Inclusion of such information is not only unnecessary but wastes valuable time. Focused, rather than “shotgun,” answers are better answers.
Having first thought about the questions, reread them carefully
and answer them.
Now having answered the question for yourself; let’s examine 3 actual
answers (left hand column) and marginal comments (bold type, right hand
column) on the following pages. The answers have not been edited
for grammar or spelling.
a. Consider the lipid molecules illustrated, identify their parts and
briefly describe how this type of lipid organization is stabilized.
|
Answer
|
Comment
|
| Example 1. The hydrophilic “heads” of the lipids are oriented towards the membrane surfaces, while the hydrophobic hydrocarbon “tails” interact to form a nonpolar inner layer. This type of lipid organization is stabilized by 2 forces: 1) Van der Waals forces—individual dipoles between hydrocarbon tails, 2) the exclusionary force of water forces the “burying” of the hydrocarbon tails. |
A good start! More specificity is required:
define “heads” and “tails” of the phospholipids
to convey understanding of the lipid structure and their contribution
to stabilization. The stabilization forces are correctly identified
but are not clearly related to the membrane components. Nor are
they adequately described. |
|
Example 2. Lipids are amphipathic in nature, having polar heads (hydrophilic) and nonpolar hydrocarbon tails (hydrophobic). The lipids in the bilayer are arranged so that the polar hydrophilic heads face the polar cytoplasmic and exterior sides of the membrane with the nonpolar hydrophobic tails point toward each other and away from polar regions. This arrangement was proven by the Languimir trough experiment where lipids were dissolved in a nonpolar solvent which was placed on a film of water and then the solvent evaporated leaving the hydrophilic heads closest to the polar water and the tails (hydrophobic) pointing into the air. |
The components of lipids are first defined followed by a description of how the lipids are arranged in the membrane. In the last sentence of the paragraph, experimental
evidence for membrane structure is included although the question
didn’t specifically ask for it. It is irrelevant in this
context. |
| This type of lipid organization is stabilized by cholesterol, polarity, and Van der Waals forces. The cholesterol acts as a mortar to fill in gaps between lipids thus stabilizing them. Polarity insures that hydrophobic heads will point toward each other, establishing a nonpolar environment. Van der Waals forces maintain the binding of the lipids. If the tails are saturated then Van der Waals forces are strong because of tight packing of the tails. |
The first sentence is succinct and focused, but
the following sentences are wordy and don’t clearly explain
how “polarity” and Van der Waal interactions work.
The importance of water on either side of the membrane is ignored. |
| Example 3. The lipid molecules seen in this diagram are phospholipids. The parts shown in this diagram are the phosphate head and the two hydrocarbon tails. The phosphate head is the round part on the outer membrane. The tails are the squiggly lines coming from the head. The glycerol molecule is not shown in this diagram. It is the link between the head and the tails. | This answer first describes the diagram using specific language to define parts of the lipid (phosphate head, hydrocarbon tails). |
| This organization is stabilized firstly by the amphipathic qualities of the lipids. The heads are polar. The tails are nonpolar. Thus, the two do not attract each other. Rather there is some attraction for the same kind. Therefore, heads are attracted to heads, tails to tails. Once this arrangement is established, molecular forces begin to further stabilize the structure. The chains are held together by Van der Waals forces between atoms of adjacent chains. | The sentences
used to describe how the lipids are stabilized are simple and progress
logically from one thought to the next. Actually, they heads
of the PL are “attracted” to water dipoles and repelled
by each other. A “much better” more focused answer! |
a. Indicate clearly which side of the membrane shown is exterior and which side faces the cytoplasm, and briefly defend your designation.
|
Answer
|
Comment
|
| Example 1. Carbohydrates are attached to the exterior surface only. | Short and
sweet, and an excellent answer! But…the student failed
to identify the carbohydrates in the diagram! Resist the temptation to fill all the available space with an answer! |
| Example 2. The
side with the carboxyl group (COO-) and the amine (NH3) face the cytoplasmic
side because the transmembrane protein passes the membrane eight times,
making both ends remain on the cytoplasmic side. If the transmembrane
protein passes an odd amount of times, then because of polarity, the
polar COO- group end will remain on the polar exterior and the nonpolar
NH3 remains in the relatively less polar cytoplasmic side. A
group of molecules attached to the transmembrane proteins are found
on the exterior of the membrane because they are used to help erythrocytes
bind to one another. |
The location of the amino and carboxy terminals has no necessary relationship to which side of the membrane faces the cell’s exterior. The cytoplasm and the cell’s exterior are
aqueous and equally polar. Too much memorized detail, poorly
related to the question. What is the “group of molecules”? Function information irrelevant! A very long, illogical and badly “garbled” answer. |
| Example 3.
On the diagram, the region below the membrane is the exterior.
This is made clear by the branching carbohydrate attached to the integral
protein. |
This question is concisely answered by reference to the diagram, providing supportive evidence. |
At this point you should critique your own answers to the questions and discuss them amongst yourselves. You could also try modifying the problem with additional questions of your own: for example, what level of protein organization is depicted in the box identified by the arrow? what is the name of the specific protein structure in the box? What is the function of Band III protein, and how is its overall structure related to that function?
Now on the next page, consider a more complex problem concerning plasma membrane organization? Note in particular that some questions refer to how these proteins are synthesized, a topic that you may not have covered yet. Not surprisingly, this question was taken from a final examination. Try answering those parts you can, now, and return to the question as the course progresses.