Preface | Introduction to Problem Solving | Problem Sets | Acknowledgments
| D.4. Insulin <<Previous Problem Next Problem>> | |
4. (26 pts) Consider the synthesis of insulin, a protein hormone with
molecular weight of
approximately 5.8 kDa which is secreted by endocrine cells of the pancreas
in response to
elevated blood glucose levels. The functional hormone consists of two
subunits, “A” with a
molecular weight of 2.3 kDa and “B” with a molecular weight
of 3.5 kDa, linked by
disulfhydryl bridges; the tertiary structure of the A chain is also maintained
by an
intramolecular disulhydryl linkage. The primary amino acid sequence of
the subunits and the
location of the disulfhydryl bridges is illustrated in the figure below.
Reduction of any of the three disulhydryl linkages inactivates the hormone;
neither subunit by
itself exhibits any insulin activity.
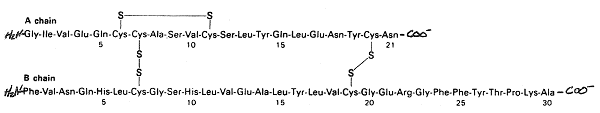
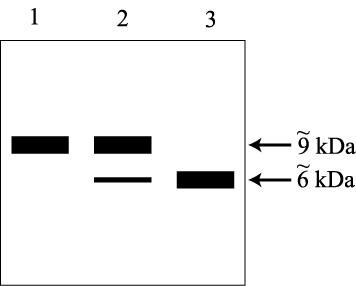
To examine the synthesis of insulin, homogenates of the endocrine gland
were fractionated
into organelle components and examined by SDS-PAGE (under oxydizing conditions
that
preserved the disulfhydryl linkages). The presence of insulin in each
of these fractions was
then determined using a radioactively labeled antibody specific for the
hormone. An
autoradiogram of the gel is reproduced below: each band represents the
binding of the labeled
antibody and a band’s molecular weight is indicated in the right
hand margin. (The technique
is called a “Western” and the additional procedural details
are irrelevant here.) Consider
these data and what you know about protein synthesis, and answer all the
following
questions.
|
Lane 1: rough microsomes Lane 2: Golgi vesicles Lane 3: Secretory vesicles |
 |
A. (4 pts) Insulin is not a glycoprotein, although other Golgi-processed
proteins produced by
these glands are. How come?
Before (or after) you answer this question, consider the following incorrect
answers. Several
are not so much wrong, as inappropriate, because they address why
insulin may have evolved
the way it did and not how it’s synthesized the way it is!
Put another way, some answers
provide an “ultimate” not a “proximal” reason for
insulin to have the structure it does. They are all quite imaginative
answers, however! Some important features of the following answers
to Question A. are irrelevant (beside the point) and - this should
be the give-away – some rely
heavily on information extraneous to BI250 text or lecture. Also, some
of the information,
relevant or not, is simply wrong!
|
Example 1: Insulin is not a glycoprotein because
it is secreted in response to elevated levels of glucose which means
it must be stored in the cell prior to release. It is known that
glycoproteins are not found in the cytoplasm and therefore stored
insulin must not be glycosylated. Example 2: Because the Golgi-apparatus is also responsible
for the removal of glyco residues and due to the very nature of
insulin. It breaks down saccharides it is unlikely to be glycosylated. Example 3: Insulin is secreted as a “response to
elevated blood glucose levels.” If it were to have oligosaccharides
attached to it, it would not lower the sugar concentration in blood
to homeostasis as it is designed to because it would be adding sugars
into the blood. As a result, disulfidryl bonds help form the tertiary
structure instead of oligosaccharides. Example 4: ...Insulin may not need to be glycosylated to form its 2º, 3º, and 4º structure... |
What’s wrong with the following answer?
| By looking at the molecular weights it appears that insulin possibly begins in the ER with a second (a) subunit. In the Golgi, however, rather than odifying the protein with sugars it removes a b-subunit and disulfide bonds become internal to the a-subunit. Other proteins are modified to become glycoproteins by the addition of sugars. |
So what’s a good answer? Here’s an excellent one (from a student
who ultimately earned an
A+ for her final grade)!
| It is likely not a glycoprotein because it does not have the correct amino acid sequences that would code for the addition of either an N-linked or O-linked oligosaccharide. For example it does not have the –Asp-X-Ser/Thr- sequence that codes for the addition of an N-linked oligosaccharide While many proteins are glycosylated in the Golgi complex, the process is not random, but proceeds in response to the information contained in the amino acid sequence (and ultimately in the nucleotide sequence of the gene). |
B. (4 pts) Based on these data, is insulin the product of 1 or 2 genes? Why?
C. (8 pts) Describe the synthesis of insulin, using a diagram, that is
consistent with these
data and with your current understanding of protein synthesis.
D. (5 pts) Suggest an additional experiment that would test your hypothesized
biosynthesis
pathway and describe clearly what the results would indicate.
E. (5 pts) If insulin mRNA were translated in a test-tube, using a vesicle-free,
SRP-free but
otherwise complete cell-free translational system, how many different
product(s) would be
detected using the antibody described above. What would be its (their)
molecular weights?
Why?